- Overview
- Summary
- Funding
- Cite This Publication
Overview
Who: Jessica Krieger, Byung-Wook Park, Christopher R. Lambert and Christopher Malcuit
Published: July 11, 2018
Where: PeerJ
Key Takeaway: This cell culture recipe could be used to grow better muscle fibers and combined with other techniques to help scale-up cultured meat production.
Research Topics:
Summary
Jessica Krieger et al. clarify the role of TGF-β1 signaling in skeletal muscle myogenesis, and the specific roles of fibroblasts and myofibroblasts in myogenesis. In this study, they grow 2D and 3D monocultures and co-cultures with and without TGF-β1, and monitor changes in gene expression, cell phenotype, and elastic moduli of the tissues. The study finds 3D co-cultures of myoblasts and myofibroblasts treated with TGF-β1 are most effective at driving myogenesis and myotube formation to form the most stable, organized tissues. The results pertaining to the impacts of TGF-β1 are consistent with similar studies, though Krieger et al. acknowledge myofibroblasts are superior to fibroblasts in driving myotube formation. Furthermore, Krieger et al. show the use of a hydrogel-based system for helping to scale-up this process. The study offers an attainable cell culture model for cultured meat production, highlighting that TGF-β1 treatment and co-culturing with myofibroblasts enhances myogenesis.
Written by Morgan Ziegelski
Cite This Publication
Krieger, J., Park, B.-W., Lambert, C. R., & Malcuit, C. (2018). 3D skeletal muscle fascicle engineering is improved with TGF-β1 treatment of myogenic cells and their co-culture with myofibroblasts. PeerJ, 6, e4939. https://doi.org/10.7717/peerj.4939
You Might Also Like...
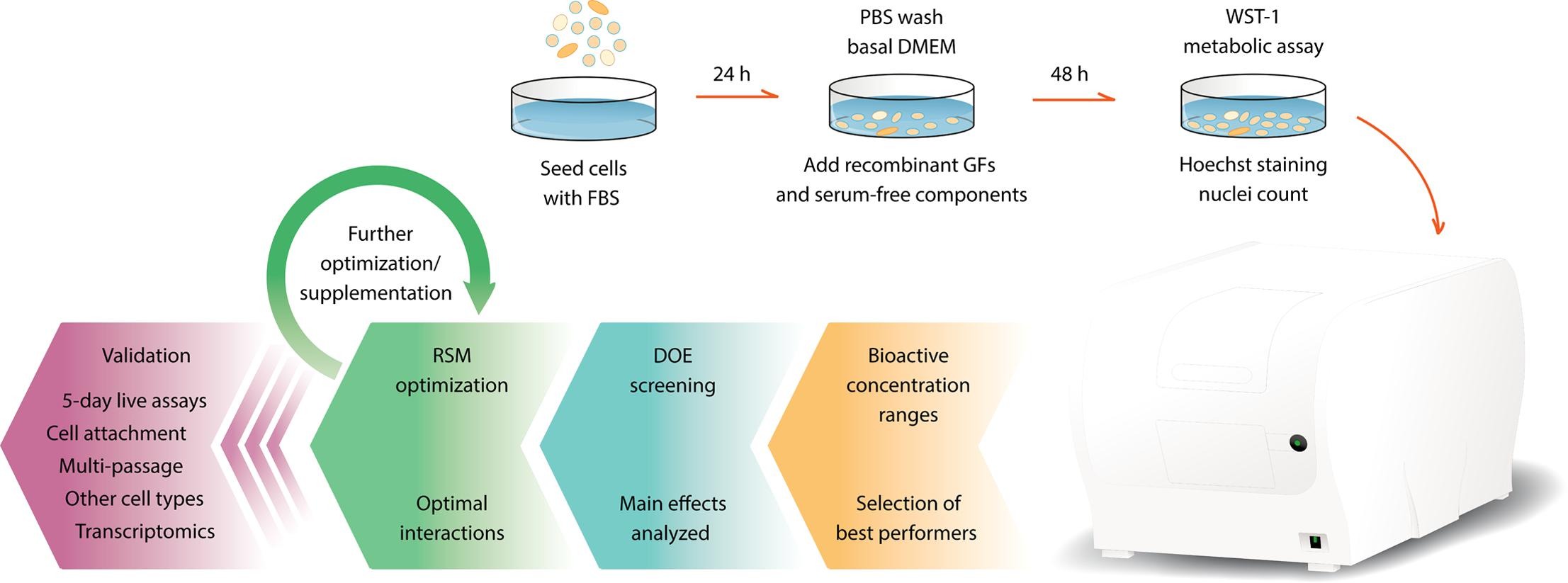
A simple and robust serum-free media for the proliferation of muscle cells
Stig Skrivergaard, Jette Feveile Young, Navid Sahebekhtiari, Cameron Semper, Meenakshi Venkatesan, Alexei Savchenko, Peter J. Stogios, Margrethe Therkildsen, Martin Krøyer Rasmussen
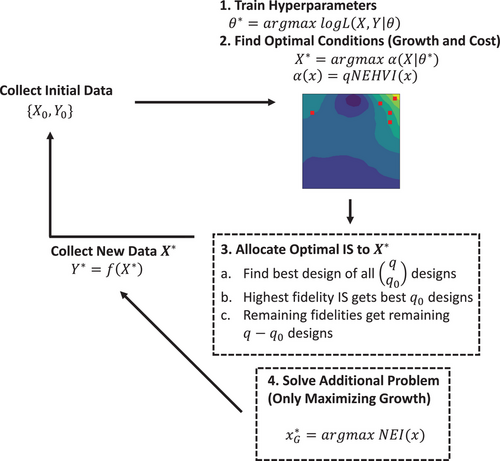
Multi-objective Bayesian algorithm automatically discovers low-cost high-growth serum-free media for cellular agriculture application
Zachary Cosenza, David E. Block, Keith Baar, Xingyu Chen
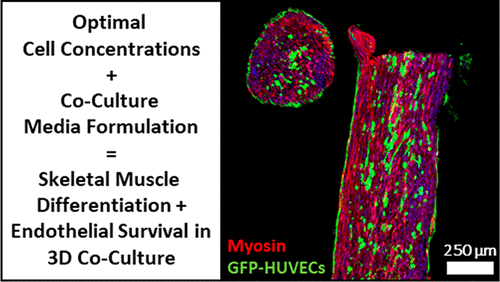
Optimization of Culture Media and Cell Ratios for 3D In Vitro Skeletal Muscle Tissues with Endothelial Cells
John SK Yuen Jr., Brigid M Barrick, Hailey DiCindio, Jaymie A Pietropinto, and David L Kaplan



