Transcript
Also available on Spotify, Apple Podcasts, or wherever else you listen to podcasts!
Alex (00:04):
Thanks for joining us for the Cultured Meat and Future Food podcast. On this episode, we’re excited to have Scott Allan as part of the New Harvest Fellowship series. Scott Allan is a New Harvest Research Fellow and PhD student with the Center of Sustainable Chemical Technologies at the University of Bath in the UK. He’s working with Dr. Marianne Ellis and Dr. Paul De Bank on bioreactor design for scaling up cultured meat production with a focus on hollow fiber bioreactors. Prior to this, Scott obtained his Master’s degree in chemical engineering from the University of Bath and has work experience in both upstream and downstream oil and gas. His senior year project was an engineering design project with individual and group aspects. Focusing on the design of hypothetical large scale production plants for cultured meat production. He is now actively pursuing research for a sustainable future in the Ellis lab with an interdisciplinary approach at the intersection between tissue engineering and bioprocess design. There’s a lot to learn on this episode. So grab your notes and let’s jump right in.
Alex (01:07):
Scott, welcome to the show.
Scott (01:09):
Hi, Alex. Really happy to be here. Thanks for having me.
Alex (01:13):
Scott, please tell us a little bit about your studies before getting involved with New Harvest and really how you were introduced to the New Harvest Fellowship.
Scott (01:20):
Yeah. So I did my Masters in chemical engineering at the University of Bath. During my degree, I did two placements in oil and gas. One was a summer internship in upstream oil and gas and the second one was just over a year long in downstream oil and gas where I basically worked on an oil refinery. But I always had more of an interest in bioprocess engineering and the biological side of things. So in my final year of my degree I took a course in biomedical engineering where we basically looked at processes for tissue engineering and regenerative medicine. During this course, my lecturer at the time mentioned that we can use these techniques and these skills that we were being taught to actually produce food in a more sustainable way. And this really piqued my interest. So I then went and started researching and I came across the New Harvest website, which is a great source for information on cellular agriculture. and while I was doing this, I saw that they had an opportunity to apply for a Fellowship where you can basically do a funded PhD within this space. So I was able to put together a proposal and apply to basically work on scaling up bioreactors. Thankfully, I was awarded this opportunity. I was able to combine this with funding from the Center for Sustainable Chemical Technology. So now I’m able to spend my time working in the lab towards my PhD.
Alex (02:44):
Wow, that’s cool. And so you mentioned upstream and downstream of the energy companies, oil and gas companies. Can you tell us a little bit about very generally what upstream means, what downstream means and what it means for gas and potentially what it could mean for cultured meat production?
Scott (03:04):
Sure thing. So the terms are probably used a little bit differently in oil and gas compared to biotechnology. In oil and gas, upstream would mean the actual extraction of the oil or the gas from the reservoir in the ground. And then the downstream would be refining the crude oil into the products that we use, like petrol. But if you think about biotechnology. In terms of cultured meat, upstream will refer to the actual expansion and growth of our cells and potentially also the differentiation phase when we take the stem cells and we guide them into the desired tissue products or the desired tissue. And then the downstream will be when we take the tissue and then we formulate it into our final product, whether it be a burger, sausages or steak.
Alex (03:56):
Okay, cool. And to kind of step back just a little bit in terms of that process, can you kind of walk us through the basics of cell cultured meat production and in terms of the work you’re doing, which aspect of cell cultured meat production are you most focused on?
Scott (04:17):
Of course. So if you think of the meat we eat in terms of its components, it is primarily comprised of muscle and fat tissue. Now, as an engineer, when I think of meat as a product, what we want to do with cultured meat is essentially change the way that we produce this product. So rather than growing a whole animal to get this final product, what we want to do is grow the meat outside of the animal. So the general idea is that you would take a biopsy from your desired animal or fish species and isolate the stem cells that you want to grow. And then within a bioreactor, you would expand or proliferate those cells to increase the number of cells that you have. And then you would mature these cells or differentiate them into your desired tissue type, whether it be skeletal muscle or fat.
Scott (05:04):
Now, most people within the industry will agree that there are essentially four different technical challenges or areas of focus that require research and development. So these include the cells, the scaffold used (as most mammalian cells are adherent and require a surface to attach to), the media, which is essentially the nutrient rich broth that we feed the cells, and then the bioreactor and overall bioprocess design. So the bioreactor is essentially a controlled environment that we use to grow the cells in vitro to keep them alive and happy. Now, my research is focused primarily on the bioreactor and bioprocess design side of things, this controlled environment within the bioreactor includes parameters such as the temperature, pH, dissolved oxygen and carbon dioxide, and also the nutrient supply. So my research is focusing on the bioreactor and what we want to do is move from the lab scale to a production plant process.
Alex (06:03):
So when we’re talking about process plants or really taking cultured meat production to that next phase, I guess first off let’s think about the companies that are announcing that they’re going into the next phase. So we’ve got Memphis Meats has released the documentary. Have you seen the documentary from Memphis Meats?
Scott (06:23):
No, I haven’t had the opportunity to yet.
Alex (06:25):
Oh man, it’s great. So they kind of show the path from the starting point (and it’s really cool how the documentary team was there in the early days) then to their growth. And what’s interesting is like near the end – and it ended like sometime last year, so they’re farther along now,- but in the end you see them kind of like touring these big facilities that could be like their next production plant. But you know, you have Memphis Meats, I think JUST has announced that they have some larger facilities. Blue Nalu is working on some new facility plans or designs. What does it really take to go from the lab scale to the production scale? And maybe you can tell us a little bit about pilot scale, what that actually looks like in terms of how much space you need and what it could look like in the future. Can you shed some light on that?
Scott (07:20):
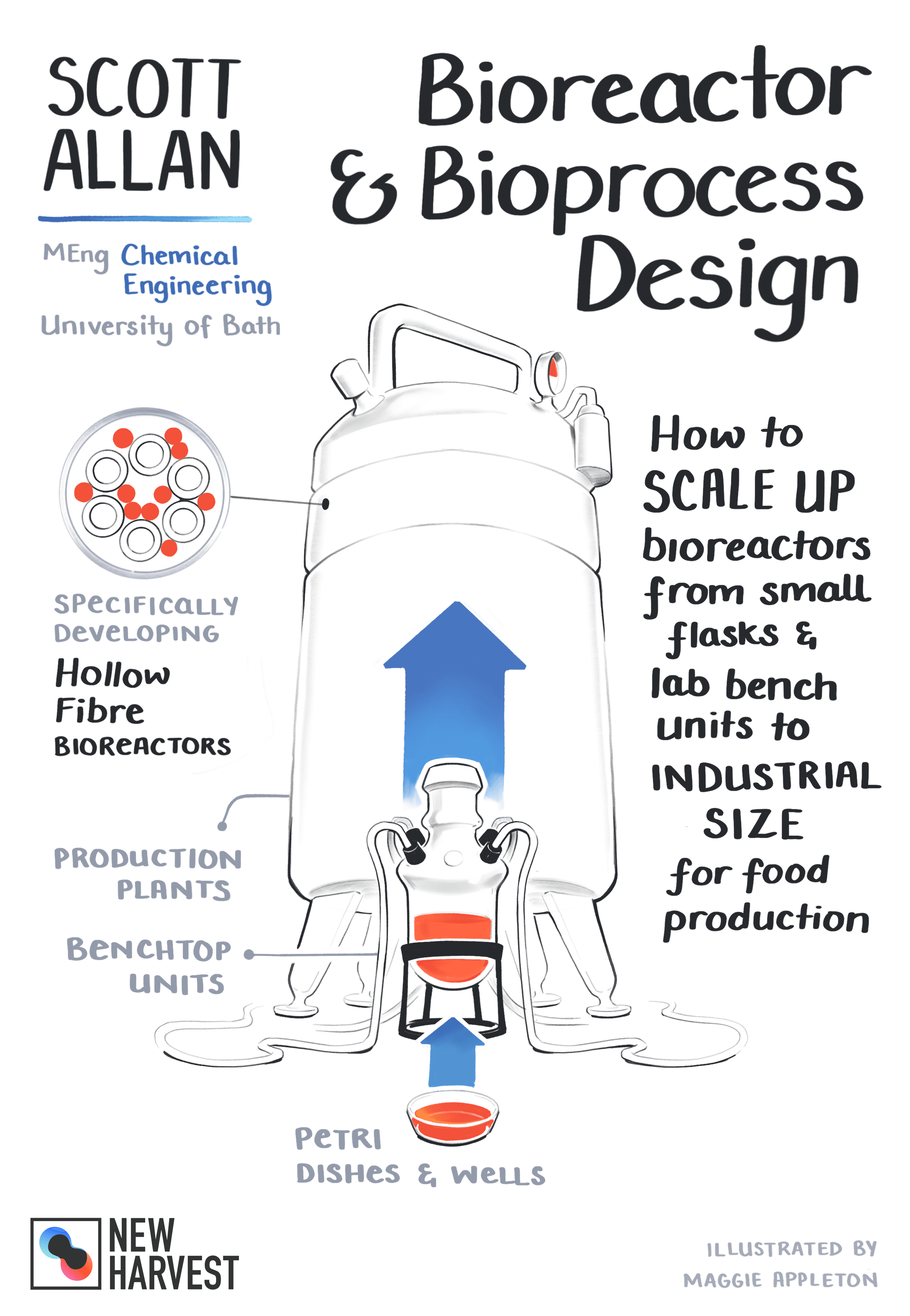
Of course. So the general steps in scaling up from lab scale to a full production plant would normally be starting with your cell culture at the lab scale, where you’re doing everything in tissue culture flasks and wall plates. You would then move to bench top bioreactors. These are ones that can literally just almost fit on the top of your kitchen cabinet. If you’re thinking about the common stir tank bioreactor, this would be anything about 250 milliliters to maybe five liters. And then you want to, ideally you want to be moving towards a pilot plant. Now pilot plant, as a term, does vary. And in terms of the size that it refers to does differ for different industries and it’s not set in stone. It will very much depend on the type of bioreactor you’re using. But the general idea is that you’re moving to a larger scale and typically something that would maybe take up a whole room. And what you’re trying to do is see whether all the parameters that you’ve optimized at the lab scale or benchtop scale, whether they scale linearly or exponentially, whether you’re able to transfer everything and still get the same results before you start putting in a lot, much larger capital investments to actually then go to full production plant scale. And production plants scale might be, if you were to think of say a brewery, multiple thousand liter fermenters or stirred tank bioreactors.
Alex (08:52):
From an engineering standpoint, what is a project of that size actually look like? I don’t know if you have experience with this back in the oil and gas, but what kind of project is that? Right? Like, so if a cultured meat company was ready to go to like a brewery, the size of, I don’t know, one of Heineken’s breweries, what would that look like? Would they be bringing in a consulting team and then a construction team? What is the overall process? And I guess I asked this in a more of a generalized way.
Scott (09:24):
Yeah. One will depend on the size of the company and who they’ve hired internally. So like actual expertise of their employees. So whether they actually do a lot of the detailed design in house, or if they do outsource it to consultant companies, what you sometimes find is that the company, or say one of the cultured meat companies, will do some of the preliminary design. They’ll have like a desired product throughput, amount of product that they want to produce maybe in kilograms per year or tons per year, and they will roughly size some of the units, so like the storage tanks, the bioreactors, the heat exchangers. Then they would outsource it to consulting companies who would then do the detailed design on like a single unit, so like just one of the heat exchanges or one of the bioreactors. And then that would then be passed on to mechanical engineers who would be involved with the actual construction of it. And then there’s the whole steps of actually bringing it together and putting it together.
Alex (10:26):
This is super exciting. So we just kind of asked about literally what it looks like from a size standpoint to go from benchtop to pilot scale. And so now for the research that you’re doing, what kind of lab environment are you in on a day to day?
Scott (10:41):
Okay. So here at the University of Bath, I’m working in the Ellis lab. The Ellis actually specializes in bioreactor design for tissue engineering applications with some of the past work done by students focusing on a range of different tissue types, such as bone liver, and also immune research. My research, as mentioned, is focused on cultured meat. What I’m doing is defining some of the fundamentals that we need to know to scale up the production of cultured meat, but focusing on a type of bioreactor known as a hollow fiber bioreactor. And I’ll talk about hollow fiber bioreactors in a sec, but just to take a step back, within my lab at the University of Bath, there are currently two PhD students. And I’ve also had a number of students who have worked with me in the past and Marianne my PI actually supervises and runs a final year chemical engineering project every year where a group design a hypothetical cultured meat production plant.
Scott (11:52):
So the University of Bath has actually become a bit of a hub for cultured meat research in the UK. I mean, another example is that there was a previous PhD student who actually worked with my co-supervisor Dr. Paul De Bank and his work was focusing on edible scaffolds. So it’s really exciting to see so much cultured meat specific research happening at the University of Bath. And it’s not just the technical side of the research, but also on the consumer acceptance side of things. Chris Bryant did his PhD here at the University of Bath as well.
Alex (12:29):
Great, it’s cool to see actually so much going on in one area, because I think like you said, there were other students that you’ve worked with. What’s cool about that is that it gets other people interested and excited once they hear about some of these projects that are going on, hopefully kind of having a little bit of a domino effect or at least one can be hopeful about having a domino effect. So I want to ask you a little bit more about hollow fiber bioreactors specifically, but can you first tell us, and you briefly mentioned it earlier, but can you please tell us a little bit more about the different types of bioreactors that there are today, either how they operate or what they’re called and why they’re different?
Scott (13:10):
Of course. So there are many different types of bioreactors available that are used in a wide range of different industries for different applications, such as brewing pharmaceutical drug production and wastewater treatment, but the best parallels to other biotechnology fields that we should probably be looking at is therapeutic protein production, when mammalian cells have a host and also the cell based therapy industry or regenerative medicine. So some common types of bioreactors include stirred tank bioreactors. This is probably the most one that many people may be able to understand or picture when they think of a bioreactor cause these are the kinds that are used in beer brewing and fermentation. These are essentially large stainless steel vessels with an impeller inside that mixes the contents. You can also get rocking bag bioreactor sometimes called a wave bioreactor where a single-use bag is rocked back and forth, creating like a wave internally with the media.
Scott (14:16):
Then you also get fluidized bed bioreactors, where you have the liquid flowing in continuously from the bottom. And this liquid flowing upwards actually keeps the cells in suspension. And I can list quite a few more types of bioreactors. You’ve got airlift bioreactors, packed-bed bioreactors, rotating wall bioreactors, and vertical wheel bioreactors. So you can see that there are lots of different bioreactor types to choose from, but all of them have their own associated advantages and disadvantages. So when you are actually choosing a bioreactor type, some of the things that you might want to consider include the operation mode, whether you operate it in batch mode. So you put all the media in and you just leave it. So the cells will slowly consume some of the substrates and we’ll be producing some byproducts, which might actually be toxic. Or fed batch, where you add a little bit of media, a little bit of fresh media over time, or you supply the media continuously so you have a constant continuous stream of fresh media and while removing all the media at the same time. But with that, you might end up depending on the bioreactor type, you might actually end up removing some of the cells as well. Then you also need to consider things like the shear forces that are created, for example, from that rotating impeller or the rotating wall of the bioreactor and things like gradients within the actual reactor. So these could be gradients related to substrate concentration or concentrations of the different nutrients. Thermal concentration, so like a change in temperature throughout the height of the rector or the width of the reactor, any of these gradients could potentially have a negative effect on cell viability or the way that the cells grow within different areas of the bioreactor. And then you also need to consider the method and impact of scaling up, which often dictates the maximum size that the reactor can be for a given application.
Scott (16:08):
For example, for some of the reactors, for every increase in the vessel length or height that you make, you might have an exponential increase in the angle of velocity required of the actual impeller or the rotating wall. So I’ve mentioned a lot of reactors here, but one of the key points for cultured meat production is that with this being such a new nascent field is that we don’t currently know which bioreactor type will be the best option to use. And so much of the research that is required within the area of bioreactors will essentially be on testing the different types of bioreactors with different types of cells and different cell species and comparing them. And another consideration is whether the bioreactor will be used for just the proliferation phase or just the differentiation phase, or if you want to use the bioreactor configuration for both. Did that make sense, Alex?
Alex (17:01):
Yeah, no, that was great. And another question I have is based off of what we were discussing earlier, would that mean downstream process would be extracting cells from a bioreactor, in our case?
Scott (17:14):
So the downstream system will depend entirely on the final product form that you are trying to create. Whether it be a processed meat product or a full cut piece of meat, or say a dry powder source of protein versus a wet cell biomass that you want to put it into like a hybrid product. So this will dictate some of the downstream units, such as whether you have a chopper, a dryer, units for flavoring and texturizing, your packaging and labeling, all of these for under the realm of downstream. And what’s actually interesting is that within a traditional biotechnology process, say for example, the production of recombinant proteins or therapeutic proteins, the downstream would actually refer to a lot of the separation units that are used to purify the product. Now for us, that might be necessary, but it might not. One thing that that will depend on is whether your scaffold that you’re using is edible and in which case you might want to leave it in the final product. Or if it’s not edible, is it biodegradable? So do you then need to break it down so that it’s not in the product. Or do you need to actually remove the cells from the scaffold? In which case you might then require some purification units to actually just get the cells out. But another consideration which does cross over and blur some of the lines between upstream and downstream is that if we are using a perfusion bioreactor or supplying the media in a continuous operation mode, you might require a recycle, in which case you might want some downstream separation units to actually retain some of the valuable nutrients and return them to the system.
Alex (18:56):
I see. Okay. So going back to hollow fiber bioreactors. Tell us about hollow fiber bioreactors and more importantly, in like an ‘explain it like I’m five’ type of way. Tell us why these are interesting for applications of cultured meat.
Scott (19:13):
Okay. So I mentioned previously a number of different bioreactor types, and then also the operation mode, essentially how you can supply the media to the cells within the bioreactor. So if we think back to the continuous operation mode where you’re putting fresh media in and taking out the used media, hollow fiber bioreactors are what we call perfusion bioreactors. Perfusion by definition means that you’re continuously supplying the media, but retaining the cells within the system. So this has beneficial because we don’t actually lose any cells in the outflow, that effluent flow of media. So the way that a hollow fiber bioreactor works, I guess I’ll just take a step back and explain what it looks like for anybody who isn’t quite familiar with the term. If you imagine taking a handful or a bunch of drinking straws and putting them in a cylinder and then laying it down horizontally, that’s roughly what a hollow fiber bioreactor would look like. So those straws are like the hollow fibers, which are the scaffolds that the cells attached to and the cells normally attach to the outside of the straw or fiber. Then what we do is pump the liquid media through the middle of the straw or fiber. And because the fiber wall is porous, the media is able to permeate through the wall to the cells growing on the outside. So this has a number of benefits. One of them is that it allows us to decouple the cells from the shear stress of the flowing liquid. And then secondly, it allows us to overcome some mass transfer limitations, which essentially means that we can supply the cells with the nutrients in a very efficient manner. And this allows us to achieve a high cell density, which I’ll talk about a little bit more in a bit.
Scott (21:06):
But this efficient means of mass transfer is because what we’re doing is essentially mimicking the vasculature within our bodies. So this is important because there is something called an oxygen diffusion limit, which is about 200 microns. And beyond that, oxygen would not be able to get to the cells. So in our body, all of ourselves are within this 200 micron limit to a capillary. And so they have access to oxygen. So within the hollow fiber bioreactor, when we are able to keep the cells within 200 microns of a fiber, it means that all the media that’s permeating through the wall with oxygen, this oxygen can actually get to the cells. If the cells start growing or are placed further away than this 200 micro limit, they then become starved of oxygen and will eventually die. So by being able to keep the cells within this oxygen diffusion, we can have the cells grow to a very high cell density within the bioreactor. And this is one of the major advantages of hollow fiber bioreactors because this essentially means that we can achieve the same number of cells within a much smaller bioreactor or a much smaller size reactor vessel. So as you can imagine, this has the benefits of reducing the floor space requirements, and also potentially reducing the energy required to maintain that reactor at isothermal conditions or a constant temperature for the cells.
Alex (22:31):
So is all of your work related specifically to hollow fiber bioreactors?
Scott (22:36):
Well, not entirely, actually. So I mentioned earlier some of the different areas for focus in research and development or technical challenges, which are cells, scaffold, media, and bioreactors. But what I think is important to mention is that all of these are actually interlinked. Any decision that you make with regards to one does impact some of the design related to the others. So for example, I’m using hollow fiber bioreactors, and that actually dictates the form of the scaffold that I need within the reactor. So as part of my work, I also produce the hollow fibers that are used within the reactor in house. And this is a very cool process called fiber spinning, but those aren’t the only scaffolds that I make. I also have done some work on using decellularized grass as a scaffold. So this has been some very exciting work. And the original idea actually came from my co-supervisor Dr. Paul, De Bank. And what we do is we strip the grass of its natural cells and we’re left with the cellulose backbone and they are these beautiful ghostly looking pieces of grass or grass blades that are nice and transparent. And this work is actually using a decellularization procedure that was adapted from the one used in the Gaudette lab at WPI. And Alex, I know you actually recently interviewed Jordan on the podcast where he was using decellularized spinach leaves. So it’s a very similar process to what he uses. And I hope that I’ll be able to talk about the decellularized grass and the work that I’ve been doing in a bit more detail very soon.
Alex (24:12):
Very cool. How has bioreactor design changed in recent years and what kind of advancements will be made to bioreactors specifically for the use of tissue culture or cultured meat?
Scott (24:24):
That’s certainly a very interesting question, Alex. And to be perfectly honest, I would say that the main reactors that are currently used in cell culture industries, such as the stirred tanks and rocking bags, haven’t actually changed that much in terms of their outer appearance, but they have been optimized with a lot of smaller internal changes. So a lot of research has gone into things like adjusting the type of impeller and rotation speeds, which allow them to reduce the local shear forces that are exerted on the cells. And another thing that’s been very interesting development is the coupling of these reactors with cell retention devices, whether it be external or internal allows the use of these bioreactor types to be operated in perfusion mode. So the idea being that the media can be supplied continuously, as you would within like a hollow fiber bioreactor that I mentioned, while actually keeping the cells within the reactor. This has allowed these more common reactor types to achieve higher cell densities.
Scott (25:27):
And then another interesting change has been the development and use of single use bioreactors. So these are typically large plastic bags, which come pre-sterilized and fits inside the stainless steel stirred tank or rocking reactor vessel. So these have the advantage of reducing the downtime between batches so that you can essentially produce more of your product per year by reducing the cleaning and sterilization time and steps. However they are single use and so are disposed of afterwards. So this is an advantage for industry such as therapeutic protein production, where the actual product of interest is a high value, low throughput product. But what needs to be done is essentially a cost of goods analysis to determine whether these will be suitable for culture meat production, because we’re now dealing with a low value high throughput product. And so we need to determine whether they would be economically feasible. Plus one also needs to consider the environmental impact of their use. A lot of them are made from a mixture of different plastics, which typically in the past have been difficult to recycle. And so they may end up in landfill, but I know a lot of work in research has gone into developing ways of recycling this. So it’s a very interesting thing to keep an eye on. I guess what I’m trying to say is that we’re in a very nascent field and it’s growing so rapidly and there isn’t currently one perfect bioreactor system. It is very likely that we essentially need to evaluate all the existing bioreactor options and compare them against each other, as well as potentially make some custom modifications or alterations or potentially even design and build some completely new bioreactor configurations. There is a lot of potential for innovation within this industry.
Alex (27:23):
And so what are your plans after your research program is concluded. Are you interested in starting a company? Are you interested in joining a company, maybe going back into academia? Do you have any kind of thoughts or interests in terms of where you’ll go next?
Scott (27:40):
Well, I definitely want to stay within the field of cellular agriculture and most likely cultured meat. Just doing the PhD has definitely firmed up my love and passion for the industry. But I currently have just over a year left of my funding for the PhD, so I don’t have any imminent plans or anything set in stone. And the way that I currently view it or look at things is that this nascent field is changing so quickly. And the number of companies that have popped up in the last few years and the progress that they’ve made has just been astounding. So at the moment, I’m keeping my options open. I would be open to working in either academia or industry, and I’m just waiting to see where I feel my current experience and skillset would be best applied.
Alex (28:28):
You can get in touch with Scott on LinkedIn and learn about new harvest at www.new-harvest.org. Scott, thank you so much for being with us today on the Cultured meat and Future Food show.
Scott (28:40):
Thank you for having me, Alex.
Alex (28:42):
This is your host Alex and we look forward to being with you on our next episode.
Transcribed by New Harvest volunteer Bianca Le.
To stay up to date on New Harvest research updates and events, sign up for our newsletter.




