A culture medium is a substance containing all the essential nutrients like carbohydrates, fats, proteins, salts, and vitamins which a cell needs to grow.
As the culture medium diffuses into the cell, the cell will grow, divide and the cell line will “proliferate”.
Culture medium is an instrumental part of cellular agriculture and generating cultured tissue because it’s effectively what allows us to take a relatively small sample of animal stem cells and end up with an enough to constitute an entire cut of meat. These stem cells would then be put into a bioreactor where they are exposed to a whole bunch of environmental cues which encourage them to differentiate into the specialized kinds of cells we get in meat.
In order to supply all the essential nutrients to the cell, the culture medium usually consists of a basal mixture supplemented with extra growth factors.
Generally, these growth factors are what complicate things. Particularly with mammalian cells, when their tissue is grown in vivo (i.e. in their body), these growth factors are supplied by their own blood.
And so, in order to try to replicate this, cellular agriculture has for a while leveraged a supplement called Fetal Bovine Serum (FBS) which, as it sounds, is the blood of a cow fetus. The two issues with this is that it is a) reliant on animals, hence defeating the goal of cellular agriculture and b) expensive because it is so inaccessible.
There is no other substance that we can easily obtain that can singlehandedly provide all these growth factors, so we turn to trying to them all individually using recombinant protein production. But, at the moment, this too is crazy expensive.
This naturally leads us to one of the biggest challenges facing cellular agriculture: finding the ideal culture medium which is one that is simple, can stimulate proliferation, is unreliant on animals, is accessible and is cheap.
Essential 8 is a culture medium which came about by refining the TeSR culture medium which contained 19 ingredients and cutting it down to the eight that were deemed “essential” for cell growth. It is a serum free culture medium (i.e. doesn’t contain animal byproducts) which has been used to grow numerous different kinds of cells across many different species.
It also only costs $376.80 per litre. Now since culturing a burger, for instance will take more than one litre of culture medium, this puts our starting cost of what would be a cheap meat product at well over $400 — needless to say, this would not be able to compete with current meat products.
But still, Essential 8 remains a relatively good starting point for trying to create a culture medium which checks all the above criteria. The Good Food Institute released a report titled An Analysis of culture medium costs and production volumes for cultivated meat in which they explored 7 realistic ways to reduce the cost of Essential 8 medium enough for it to be able to produce competitive products. Here, we’re going to go over what those scenarios entail.
The point of these solutions are to be realistic — meaning they can be done with out some sort of major technological advancement — and also based on conservative estimates — meaning they’re on the cautious side.
So, what makes Essential 8 this expensive?
As we saw above, Essential 8 is made up of eight ingredients. They are,
Essential 8 = DMEM + Ascorbic Acid 2-phosphate + NaHCO_3 + Sodium Selenite + Insulin + Transferrin + FGF-2 + TGF-beta
- DMEM: 52 ingredient basal medium which comprises the bulk of the culture medium and supplies most of the nutrients
- AA2P: Vitamin C derivative
- NaHCO_3 & Sodium Selenite: Salts
- Insulin + Transferrin + FGF-2 + TGF-beta: Growth Factors
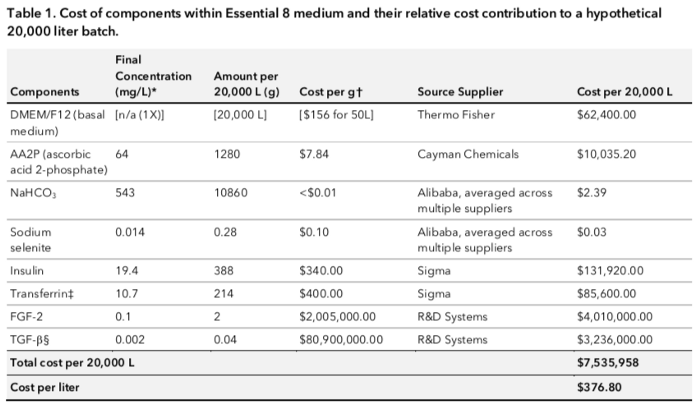
As we can see
- 99% of the cost comes from the growth factors, 96% come from FGF-2 and TGF-beta alone.
- 97% of the remaining cost comes from the basal media particularly because of the heavy reliance on an ingredient called HEPES which is a pH buffer (rebalances the pH in the presence of metabolites.
- AA2P is the next most costly contribution.
What changes can be made?
If we want to decrease the cost of the culture medium, we should start by trying to cut the cost associated with the growth factors, HEPES and AA2P.
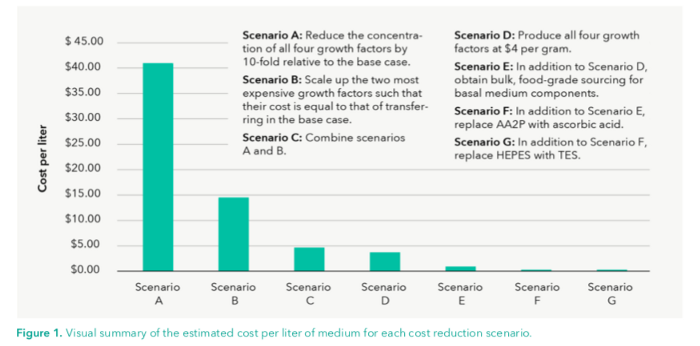
Here is a chart summarizing the resulting cost per liter of each of the below scenarios.
Scenario A: Divide all growth factor concentrations by 10.
The first solution is to just significantly reduce the amount of growth factors. At first this might seem counter productive — won’t this just hinder cell growth? But in fact it can be done by
- adapting the cell line over a few generations to tolerate lower concentrations
- engineering the growth factors to be more stable. Typically, growth factors are meant to be “short term signals”, so they degrade quickly. By enabling them to last longer, we’ll need fewer of them.
- engineering the growth factors to be more potent — i.e. more readily able to bind to the cells.
- introducing cheaper “molecule mimics” which are similar to the growth factors they are meant to replace.
Doing this will bring the cost down to $40.94 per liter.
Scenario B: Produce FGF-2 and TGF-beta at larger scales.
Right now, insulin and transferrin are produced much more cheaply than FGF-2 and TGF-beta in part because they are produced at a much larger scale. When things are produced in bulk, their cost usually falls.
If we scale up generation of FGF-2 and TGF-beta so that their cost per gram is equivalent to the cost per gram of insulin and transferrin, their costs could fall to $800 and $16 per 20,000 L, respectively. This would put the overall culture medium cost at $14.54 per liter.
Now, this seems a bit too simple. Scientifically, scaling up production should be entirely feasible because FGF-2 and TGF-beta are molecularly less complex than insulin and transferrin. However, producing the other two growth factors at this scale would necessitate some sort of initial investment in equipment and facilities as well the demand to make it practical.
Scenario C: Scenario A + Scenario B.
Scenario A and Scenario B deal with two separate aspects of the culture medium. In this way, combining them shouldn’t lead to any sort of conflict (i.e. encouraging one shouldn’t hinder the other). It’s also possible that the two will work synergistically (improvements on one front will lead to even more improvement on the other front), but that wasn’t assumed in calculating the cost.
The total cost per liter would be $4.71.
Scenario D: Produce all growth factors at $4/gram.
This would require us to produce the growth factors on an industrial scale equivalent to the way we currently produce enzymes such as lipase, cellulase and amylase. Again, this should be feasible as our growth factors are molecularly simpler than enzymes (and again, we would also need an investment in equipment/facilities as well as higher demand).
This estimate is actually quite conservative as we do produce many enzymes at a rate of 10 cents per gram, and so we might even be able to produce several times more growth factors for this cost. This brings the cost down to $3.74.
Scenario E: D + Buying DMEM components at bulk food grade prices.
Right now, the components of the basal medium are bought at a pharmaceutical grade. The cheaper alternative would be to buy them at food grade.
The concern with food grade as opposed to pharmaceutical grade in general is that there are more impurities which could contaminate our cell lines. However, with these specific ingredients, the food grade purity can be almost equal to the pharmaceutical grade purity (often higher than 99% in both cases. So, this would still be considered acceptable.
This would make DMEM’s price fall to $4600 per 20,000 L and the overall medium cost $0.85 per liter.
Scenario F: D + E + replace AA2P with food grade ascorbic acid.
AA2P is a long acting vitamin C derivative often used in cell cultures. Food grade ascorbic acid degrades faster than ascorbic acid 2-phosphate. However, it’s relative cost benefits still outweigh it’s functionality deficits. For instance, if 10 times as much ascorbic acid would need to be used in order to achieve the same effects as AA2P, the overall cost would still be 99.6% less.
This brings the cost of the ascorbic acid component down to $4.48 per 20,000L and the medium cost to $0.35 per liter.
Scenario G: D + E + F + replace HEPES with TES.
TES is a pH buffer with a similar pH range, solubility and pK_a to HEPES, only it costs half as much.
There is also the argument that we might not even need to rely so heavily on HEPES in the first place because it is most effective for cultures where the cells are frequently taken out of their CO_2 supplemented conditions or when metabolites accumulate. This is not likely in the kinds of closed culture, continuous perfusion/filtration cultures we’re considering for meat, and so we might be able to deduct even more of the cost.
Now, the cost of the DMEM component is $2,456 per 20,000 L and the overall cost of the culture medium is 24 cents per litre (a lot less than $377)!
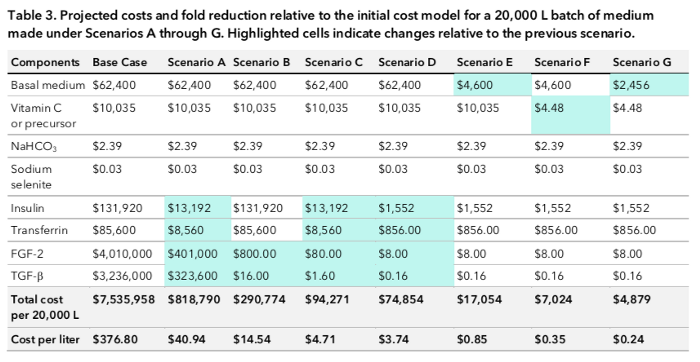
Cost per liter of each scenario
Other Possibilities
Another minor measure which might help decrease the price is just reducing the number of ingredients involved in DMEM to the essential amino acids which can not be independently produced by a cell. But, because this might stress the cells, and hinder their growth, it’s cost benefits would have to be weighed against the productivity tradeoff.
The other possibility is to eliminate ingredients which are impractical for a specific set up. For instance, DMEM contains phenol red — a colorimetric pH indicator which changes colour based on the pH. However, if the bioreactor uses remote pH monitors, this addition would be redundant and so we can save more on the cost by getting rid of it.
How much would production cost?
Now comes the question of how much producing a specific amount of meat — let’s say a kilogram — would actually cost. This depends on how much culture medium those cells require.
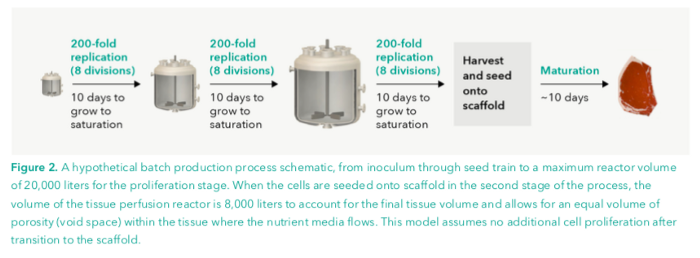
Step 1: Number of Cells per Bioreactor.
The growth configuration we’re going to be focusing on is the batch process. This entails cells being grown in batches which are all inoculated at one time for use. This is different (and less efficient) than the continuous or semi-continuous process which is more of a perpetual letting cells grow and collecting them. The reason we’re going with the less efficient process is to ensure that the estimates are not optimistic.
We’ll consider a 20,000 L bioreactor which is at the upper bound of size feasibility. While bigger bioreactors definitely exist, animal cells aren’t typically able to withstand the amount of pressure present in those models.
Cells proliferate best in what is called a seed train bioreactor, so we’ll start them off here. Once we want them to differentiate and structure onto the scaffold, we’ll put them into a perfusion bioreactor.
In a seed train bioreactor, minimum cell density requirements are considered to be 200,000 cells/ml — anything much lower will trigger cell apoptosis. Maximum cell density maximum cell density is 4 x 10⁷ cells/mL. So, in the proliferation stage, we’ll
- start the cells at minimum density
- have them proliferate until they reach maximum density
- put them into a larger bioreactor at minimum density
- repeat until we get to a bioreactor of 20,000 L.
Expanding the culture from the minimum to the maximum density will take a 200 fold increase. Since we want to start our cells from a small frozen vile, we’ll have our largest seed train bioreactor be 20,000 L, the middle one be 100 and the smallest be 0.5 L which can be filled from a 2.5 mL frozen tube.
Step 2: Amount of Time
Expanding the culture from the minimum to the maximum density will take a 200 fold increase, which is just under 8 doublings. According to Accelta, a 50–100 fold increase takes about 7 days, so doubling time is calculated to be 28 hours, making our total 8 periods 9.3 or about 10 days.
Since we have three of these seed train bioreactor phases to go through, this puts the process at about 30 days.
After, we want to seed our cells onto a scaffold so that they mature in a certain configuration which takes an additional 10 days, bringing the total time to 40 days.
Step 3: Total Yield per Batch
We’re going to consider the average cell volume to be 5,000 micrometers³ (this is a rough estimate as different kinds of cells in different species will be larger or smaller). A cubic meter of ground meat weighs about 881 kg which includes some fat.
This puts our total at
yield = (density of meat) x (unit conversion) x (avg. volume per cell) x (cell density) x (size of largest reactor)
= (881 kg/m³) x (10^-18 um/m³) x (5 x 10³ um/cell) x (4 x 10⁷ cells/ml) x (2×10⁷ ml/batch)
= 3524 kg.
Now, this all has to be put into the perfusion bioreactor for differentiation. So, we’ll round up to 4000 kg and double it to account for the necessary “void space” in and around the cells. This puts our reactor’s spatial requirements at about 8000 L.
Step 4: Required amount of Culture Medium
We’re going to calculate a couple different possibilities for how much culture medium we require.
The minimum amount we require is enough to fill the largest bioreactor which is 20,000 L. This assumes that the culture medium is never replaced.
The maximum amount we require is if we change the culture medium every 2 days which amounts to 5 medium changes for each phase or
0.5 * 5 + 100 * 5 + 20,000 * 5 + 8000 * 5 = 140,000L
Then, we can take the average medium requirement which is halfway between these two bounds at 80,000 L.
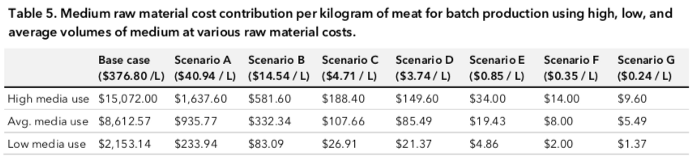
Using this, we can figure out the cost per kg of meat for each of the media uses for each of the scenarios. Scenario G, low media use is $1.37 — definitely competitive for the average shopper!
Assumptions & Oversimplifications
Now, there are a whole bunch of assumptions and oversimplifications that we’ve made throughout this process. While the assumptions can all be justified, the main oversimplifications we made which would lead to some discrepancy with the actual final result is that
- some cells will die and senesce
- some cells that will not adhere to the scaffold, and so will not be part of the finished product
- some cells won’t differentiate.
- we need to use different growth factors for different cell lines
- medium cost takes into account labour and preparation, not only the raw ingredients
- high cell density requires different medium conditions as low cell density
- other costs that go into a finished meat product in general.
But, still, in light of these potential concerns, it is indisputable that generating a kilogram of meat for less than 2 dollars is better than just under 400. The author of the Good Food Institute’s study maintained that the results were optimistic and suggest that “it is likely that cultivated meat is capable of ultimately being cost-competitive with conventional meat production at scale”.