Update 5/4/2017:
On May 1, 2017 New Harvest discontinued the research conducted by Abi Glencross at King’s College London.
A variety of situational factors made this project no longer an effective use of donor dollars. Some of the reasons for discontinuing this work include 1) an ambitious project that was not meeting the proposed timeline 2) challenges of the Research Fellow addressing a more “unconventional” approach to tissue engineering, 3) inadequate research support in this new field from the existing scientific community. New Harvest has made several improvements to the Fellowship program based on following the progress of this project. You can read our blog post about how and why we came to the decision to close Abi’s research project here.
Update 8/25/2016:
Abi is receiving the King’s College Global Research Award and will be studying under Dr. Andrew Pelling at the Pelling Lab in Ottawa Canada in 2017! Read more about her upcoming research here.
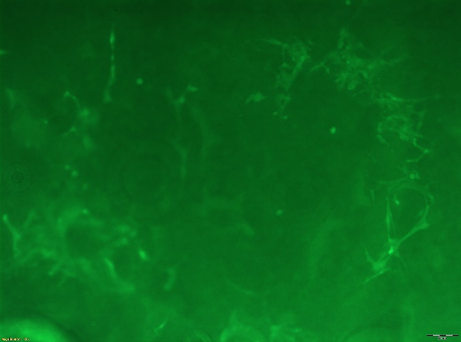
Cells in a gel! Abi is currently using a C2C12 mouse myoblast cell line to model the system (before moving to bovine). In this photo the cells are stained with Alexa Fluor 488® phalloidin, which stains F-actin and gives the cell cytoskelton shape, and Hoechst DNA stain which displays the nucleus.
The Story
Abi Glencross was in her last semester of her MEng in Chemical Engineering, ready to move on to an engineering-free life, when she was told to think about growing meat.

New Harvest Research Fellow Abi Glencross
It was an engineering design project under the guidance of Dr. Marianne Ellis at the University of Bath. Marianne applies chemical engineering principles to the design of bioreactors for large scale tissue culture. (As an aside, Marianne first heard about cultured meat in 2011, at the seminar “Tissue Engineered Nutrition,” organized by New Harvest at the TERMIS World Congress. She remembers her impression changing from “ick” to “hey, that makes sense” after seeing the seminar.)
Part of Abi’s project research was getting in touch with New Harvest. Early in the semester, at our first meeting, I remember Abi telling me up that she knew she wasn’t going to be a chemical engineer, and was instead going to pursue work in media.
Abi’s future tissue culture hood.
The day before she handed in her project, Abi sent me an email. She had found an area of chemical engineering that she cared strongly about, and now she wanted to get involved. She signed her name “Abi – Team Bath Cultured Meat.”
Two months later, Abi received a grant from the University of Bath Alumni Fund to work in Marianne’s lab. The work would be on a new biomaterial for a hollow fibre bioreactor, that could be exploited for cell expansion for cultured meat. (Hollow fibre bioreactors are by far the most energy-efficient and space-efficient system for growing tissue.)

A lab-scale hollow fibre bioreactor.
After wrapping up her summer research, Abi was hooked! She began putting together a PhD project focused on producing cultured meat, with the help of New Harvest, Dr. Mark Post, and her PhD supervisor, Dr. Lucy Di-Silvio, at Kings College London. The next thing was finding funding.
New Harvest exists because there is a funding gap for cellular agriculture research. Abi applied for grants but didn’t have much luck securing funds. So we stepped in.

Abi at her lab bench, hard at work.
Abi put together a brilliant proposal, with Dr. Mark Post as a co-supervisor, to work on the 3D vascularized muscle tissue. Work on this topic is very early and very foundational. It’s the kind of early-stage groundwork that New Harvest believes is key to building the field of cellular agriculture. Plus, cuts of meat are what drive the meat industry, not ground meat, so this research is a key first step in tackling the problem more head on. We also wanted Abi’s work to be funded because she was such an enthusiastic voice for the research. She was eager to give talks, do interviews, and encourage more people to get involved.
After weeks of working with Abi to secure funding, her proposal was funded by New Harvest donors at MaxMind. MaxMind ensured that Abi’s research would be published in an open access journal once it was completed.

Abi exploring the ability of common soy products to function as scaffolds for cell culture.
In November 2015, Abi, New Harvest Research Fellow, officially started working on her PhD project, “Creation of In Vitro Thick 3D Vascularized Skeletal Muscle Tissue for the Production of Cultured Meat.” She’s been giving talks about her work all over the UK and educating the world on what the research behind cultured meat looks like.
Read more about Abi and her work in our Getting to Know… interview.
Written by Isha Datar and Natalie Rubio, March 1, 2016
Abi’s “Diary of a Cell Farmer”
Wow, it’s been six months since the start of the PhD! How time has flown.
And guess what? I got my first muscle twitch this week! What a perfect time to look back over the already dusty notes and see where the project has come from the start. This is the real diary of a cell farmer.
Episode 1: Starting a PhD, the hurdles in producing cultured meat and those little joyous moments.
Month 1: Bambi
Starting a PhD is like a baby animal’s first steps, slightly wobbly, uncoordinated and you are convinced you can go solo but actually a bit of help stops you falling over.
The first thing I quickly learnt was cell culture 101: How not to kill your cells. Cells like to live, but they also really quite like to die. There are several ways they can die. Programmed cell death which is a natural turnover of cells (e.g. fingers form in the embryo when cells between fingers die), and necrosis as a result of infection or trauma.
Figure 1: cell proliferation assay
Trauma can come in several forms. Knocking over with your extra-long arms, accidently aspirating (removing) your detached cell from a flask (I managed to kill 40 million that way…), using a BSci students infected PBS (salt washing solution), not feeding them enough, allowing their home to get overcrowded.
The possibilities are endless. But they sure are pretty, like a cute puppy who chews through your slippers.
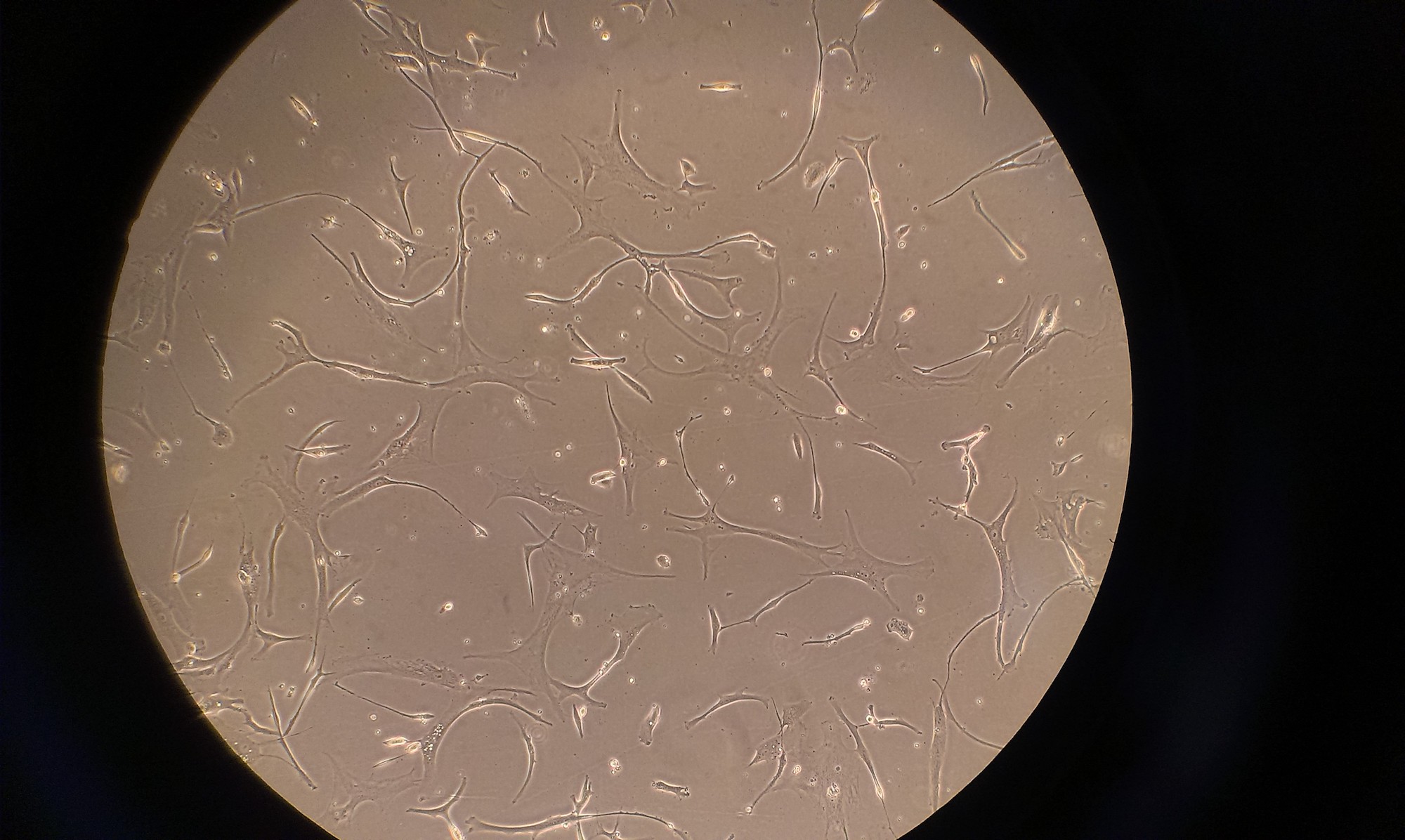
Figure 2: My first cells – mesenchymal stem cells. Mesenchymal stem cells are the precursors to bone, cartilage, muscle, and fat cells.
So I had some intensive cell culture lessons. Growing cells, changing their flasks (happy houses), seeding them on materials (they can be pretty resilient!), quantifying RNA.
I learnt very quickly that I was going to have a job on my hands to make people believe cellular agriculture is a real scientific field and my project has sound premise. Although hard, I feel this is very much making me a better scientist and be able to engage with scientists and the public on my work! I think people often think of cellular agriculture as futuristic and dystopian or ‘a solution to all our problems’ of which it is neither.
I am incredibly lucky to have such open minded and supportive funders, and this month saw me travel to New York to visit New Harvest. I was eager to learn more about how they work and develop talks and public engagement. Apparently people want to hear the diary of a cell farmer 🙂

Figure 3: Times Square
I met some amazing people on my trip. Prof. David Kaplan working on silk tissue engineering at Tufts; Modern Meadow and their amazing leather; Dr Caleb Harper and the OpenAg team at MIT.
Plus New York is amazing!!! The vegan food is out of this world, and I am not strictly vegan!
Mahnattan hotspot Wild Ginger and the most incredible indistinguishable from meat alternatives at MayWah Supermarket.

Figure 4: Wild Ginger
I came home with much more understanding of cultured meat and cellular agriculture. The hurdles, the technology and the work going on.
And I started to think ‘how can I think about creating beef differently?’. My project began with vascularization, but I quickly learnt how complex this is and this is where cultured meat differs from current tissue engineering. The tissue we make never goes into the body, so do we need blood vessels to get a product we want? Maybe we just need to perfuse our tissue? It was time to get back in the lab!
Month 2: Ideas, Ideas, Ideas
The research then became like walking across hot sand. A little sporadic, comes in leaps and bounds of inspiration and it was hard to stay in one place.
With thought of perfusion I looked to nature and natural materials. Looking to steer away from collagen and fibrin (the most widely used tissue engineering materials) as they are animal derived (ethically unstable, inconsistency between batches) I opened my mind to the materials in our world around us.
Criteria? Readily available, economic, good cell attachment, edible or biodegradable, can incorporate growth factors and nutrients for the cells, mimics natural tissue (keep cells happy), can for 3D stuctures (able to perfuse via pores or tubes).

Figure 5: Natural materials – Looking at loofa
From a cell biology point of view cells in vitro require a scaffold whether a solid support, a gel, a foam or otherwise to attach, spread and grow on (as in the body cells secrete an extracellular matrix which provide structure and biochemical cues – a large portion of this ECM is collagen hence why it makes an awesome scaffold). Thus as tissue engineers we look to use these scaffolds to model our tissue.
Hmm…porous, edible and readily available….what about meat alternatives? Tofu (soy which has been looked at for cell scaffolds), Quorn (mycoprotein) and mushrooms?
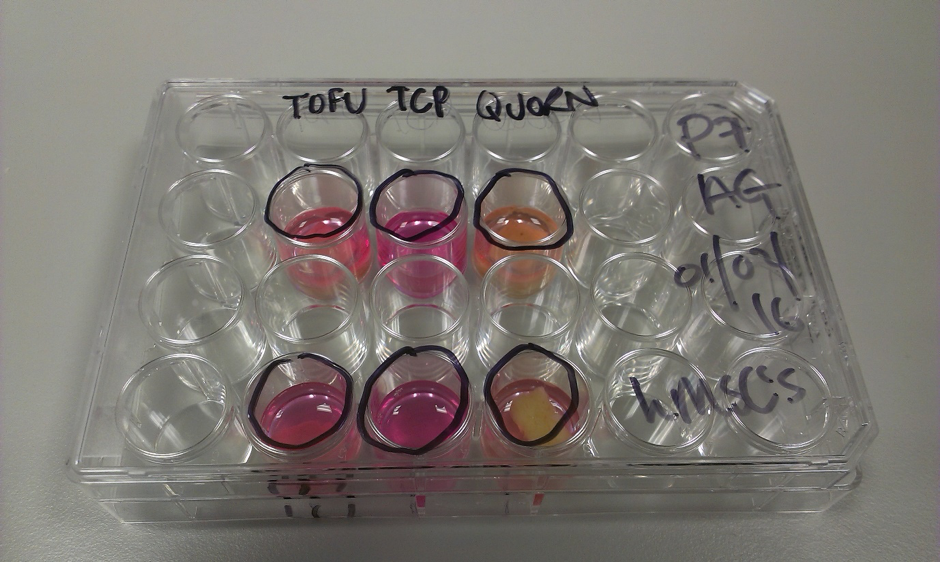
Figure 6: Food studies
Let’s have a go.
I didn’t have any of my own cells so I borrow some of my friends mesenchymal stem cells (can be converted into muscle cells or ‘myocytes’), it’s a bit like the nerdy version of swapping clothes at home 🙂
We grew them on our scaffolds, and I learnt two things.
a) We image using fluorescence (I was using a green wavelength emitting cell tracker), and tofu and Quorn are autofluorescent in many wavelengths. It means it’s a right pain to see our cells! Or do you mean not to see??
b) Actually MSC’s can live on tofu, plain and simple tofu.
Figure 7: HUVEC cells seeded on Quorn (top) vs TCP (bottom).
Okay so they don’t thrive so well on tofu as TCP which has been synthetically adapted to make for cell attachment but it’s interesting what you can grow cells on! Sadly I couldn’t image the Quorn, plus you know that vacuum sealed bag it comes in? Well I can safely say it’s not sterile in there. And mushrooms, well, it’s hard to sterilize mushrooms. The microorganisms on them are what create a balanced ecosystem in nature and they don’t really like the lab.
However you can buy a mushroom autoclave machine (high pressure steam at 121oC) which sterilizes them. Who knew? I would have thought that’s a pretty niche market…but makes another awesome experiment!
So next? How to image these materials, sterilization, more unconventional edible scaffolds, perfusion systems (honeycomb?) and maybe food industry thickeners to make hydrogels.
Figure 8: Edible gels
But it would seem cells like animal derived environments to live in. Which makes sense as that is their natural habitat. They didn’t take to our gels, but that doesn’t mean I’ll stop trying.
I also gave my first talk at The Catalyst Club in Brighton, such an amazing crowd! So open minded and lovely. I feel this is the start of the UK cultured meat conversation and it’s so exciting!

Figure 9: A tiny picture of my first talk 🙂
Oh and I applied and received a King’s College small grant for public engagement where we are looking to run an exhibition for tissue engineering, cultured meat and food at Somerset house! Woohoo!
But many of the natural hydrogels cells don’t like to live in, on, or well around. Bugger.
And I wonder if fungus could grow as a scaffold in real time and cells grow on this? Like when mycelium mapped the Tokyo train network?
Also have you ever noticed roots look like the plant version of capillaries?
Month 3: Quantification
Month 3 was all about quantifying what I was doing in the lab. Oh and going off on tangents.
Natural biomaterials turned into ‘what are the building blocks of biomaterials’, which then turned into ‘how can we make cells like these materials better’, which turned into ‘how have people done this’, which turned into synthetic biomaterials.
A small deviation, that turned complicated quite quickly. It required a lot of reading and quite a lot of time.
Conclusion? To make these biomaterials you need a lot of biomaterials knowledge! And that is a whole project in itself 😀
So I came back full circle to how can I perfuse currently established hydrogels and scaffolds, which having a side project of unconventional natural biomaterials.
Month 4: Maastricht
Okay back to the perfusion of a current system, and what better place to start than the system that made the burger. This utilized collagen to create a ‘tissue like’ gel which apparently bovine muscle grows pretty well in.
Cells like to live in collagen type I as it is the most abundant protein in the extracellular matrix. What the heck the ECM might you ask?
It is a membrane your cells secrete to provide physical and biochemical cues to support each other. I know a bit of a mouthful right? Basically it’s like the cell equivalent of…..going into a roomful of people in a jumper with pockets filled with dark chocolate and an inspiration quote on it. It’s warm and comforting, it sends signals to attract others, it supports others in the vicinity, it initiates and regulates communication, it helps to regulate your daily processes and it provides food.
Okay analogies are not my strong point. Essentially it’s super import, as are jumpers. Mimicking this in vitro is important too as cells don’t naturally secrete the ECM outside of the body.
So off I trotted to Prof. Mark Posts Lab in Maastricht for a burger making class.

I made bioartificial muscle! Woohoo! Well with a lot of help from the lab.
So I came back to London with a new system to perfuse. But first I had to recreate the current system by myself. This was a LOT harder than you would think. Troubleshooting your own work is really quite hard.
It took me a while to get this right. Another PhD learning curve.
All the while thinking how can I create channels within my system? Create capillaries? A very very complex place to start. Biodegradable channels? But then your cells die before nutrients can get to your cells and the scaffold often kills your cells or makes them seriously unhappy. Dissolvable channels? Hmmm.
Have you ever heard of a sugar scaffold? I have now. Let’s try this desktop style.

Okay so printing sugar turned out to be super hard. Sugar needs to get really hot, and when cooled it becomes a brittle structure. Precision forming at its finest.
Luckily some others in my team at King’s are looking at vascularization and bone, so we are developing a bioreactor to model this, I think there are going to be a lot of iterations!

Outside the lab we continued to design the Somerset House exhibition and I gave a few talks to students visiting King’s college. It was fun to teach them about science but also made me realise I wasn’t cool at that age, and reminded me that in the eyes of a 16 year old while dressed in my baby blue sallopettes I am not cool now. But that’s okay, I like being a nerdy meat lady.
Alex Sexton (who researches the social side of cultured meat as part of her project) and I were invited to the Science Gallery London dinner. We met some amazing people (Tristram Stuart who is waging a war on waste and is a total megababe) and had a lovely evening where all the food was waste prepared from Brixton market. Alex and I were asked to talk to Reprezent Radio (check the podcast here https://soundcloud.com/scigallerylon/fed-up-hour-show-mixdown!) chatting cultured meat and grime music. It was so much fun, and hopefully helped spread the CellAg word!
Plus I’ve developed a really deep interest in farming, agriculture and growing. Now trying to turn the warehouse balcony into an urban jungle of native plants. Hey there wild garlic…

Month 5: If at first you don’t succeed.
The Lab: In the lab it was time to fly solo and make my own BAMs! You would think this would be easy as I did it in Maastricht, however this assumption would be wrong. Flying solo is SO DARN HARD!?
Why won’t tension form in my fibres?
WHY DOES EVERYTHING KEEP DYING?
So basically I’ve been getting up at stupid o’clock in the morning to get into the lab and try and make these things. I’m hoping that I will get faster, at the moment a turtle could out cell culture me.
Darn, I’m going to have to do some serious trouble shooting as the Maastricht thin 3D system is a great place to start in creating thicker 3D tissue.
On the positive I did manage to freeze some cells for the first time which again you think would be easy, but turns out the DMSO you freeze them in to stop crystals forming kill them pretty quick too….
On the unconventional biomaterials side we explored which flourecent cell tracker we could use to see our cells. To do this we mapped the absoption and emmision spectra of our funky materials and saw what we could tag our cells with so we could see them. It was a lovely afternoon in a dark windowless room, at tleast the company was good 🙂 and I got some nice graphs to show!
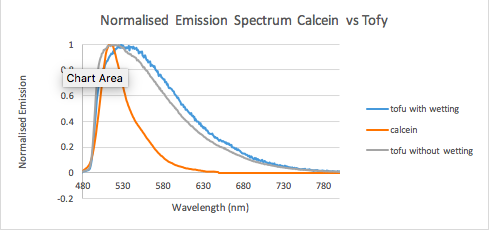
Figure 10: Calcein vs wetted and non-wetted tofu. Calcein is used as a cell tracker, and as you can see when wetted (tofu soaked) has a emission peak slightly to the right of calcein, so we should be able to see it!
Figure 11: Just imaging my food scaffolds
Written research wise I began to realise to make muscle I really had to understand muscle from the cell up. Not just whole cells but how they signal to each other and how molecules in a system effect their behaviour. I sat at my desk for many long hours writing about this (it isn’t so bad, there is a cell signalling pathway called ‘Sonic Hedgehog’ which made me chuckle…)
I’ve realised one amazing thing about being back in education is the awesome array of opportunities to develop your skills. So off I trotted to a public speaking workshop! It was so useful! From your stance (my sometimes was a little uncoordinated and fidgety) to creating a speech in 5 minutes and presenting it. I came out feeling a million dollars and ready for the next talk.
I also started a competition called ‘I’m an engineer get me outta here’ which pitted 5 engineers against each other to engage students in online forums. The winner would receive £500 public engagement money, which I was planning on running tissue engineering workshops and an exhibition. To be honest the competition was pretty tough, there was a crisp creator and a chocolate scientist! But I came 2nd which was pretty awesome, and hopefully inspired some students in the process.
I also talked at a really cool festival called ‘Fireside Festival’ and got to stay with the amazing Organizers Joanna and Jon Cherry. These guys are amazing farmers and we learn so much about beef mob grazing and crops!

Figure 12: The amazing Joe Sarah’s photo
We also have friends who run an amazing podcast where they often look at how tech and farming can work together. I had a chat with farming tech guru Abby of FARMERAMA on how I thought cellular agriculture could be used to revistalize small scale farming. Check the episode out here.
https://www.acast.com/farmerama/08farmerama
Month 6: Balance
I was privileged to be invited to talk at Future Everything 2016 in Manchester. I’ve never been to the city before and it was gorgeous! Also the conference covered an eclectic range of speakers from physicists and cellular agriculturalist to musicians and artists, which was great in discussing the future of our world. Plus my housemate got to cover it as a journalist which made it super fun! I don’t think I’ve ever had so many ‘likes’ for a photo on facebook…check out the talk and panel here.
https://soundcloud.com/futureeverything/podcast-series-life-abiaspenglencross
https://soundcloud.com/futureeverything/podcast-series-life-paneldiscussion
http://futureeverything.org/wp-content/uploads/2016/04/Life-AbiAspenGlencross.pdf

Figure 13: Looks like I’m playing tennis…
And we were featured in Bitten mag too! http://getbitten.co.uk/meat-is-made-here/
I also helped run a session on engineering and science at my old secondary school back in cornwall! It was so lovely to go back and tell kids about what I do, also help out the school that gave me such a good start and so many great years (insert warm fuzzy feeling here)
Back in the lab we are lucky to have an artist conducting her PhD. Amy Congdon who is looking at cell growth on fibres. So we are doing some collaborating, growing muscle cells on her natural and synthetic fibres to see if this aids in cell alignment.
I have also quickly understood that the life of a research involves a lot of grant writing, so my next endeavour was a travel grant which would allow me to visit San Francisco for the New Harvest cellular agriculture conference. Aaannnndddd I was awarded it! Hooray! San Fran here we come.
I made BAM’s so many time this month. You would think following a protocol would be getting easier but apparently not.
Until one fateful day I realised I had been using the wrong media, hooray!!
So I tried it again, new media.
And it STILL didn’t work. Baah!
Right let’s run an experiment deconstructing all parts of the system to see what killing the cells. The lovely long winded joy that is research. I kept going to bed dreaming of what I could do to fix it…
Month 7: Twitchy business and Cooking Up A Storm
And now it’s month 7.
They say seven is a lucky number and I believe they might be right as my bio artificial muscle worked!!!

Figure 14: It may not look pretty but it made me so happy…
And why was it not? (aside from the crazy amount of variables in biological experiments). We what was happening was I’d panic that I was being to slow in making the artificial muscle and kept reading the cell reader wrong. Too many cells for it to read, and I was putting in 10x too many in my gel.
So they often shrivelled and looked cloudy mini brains as they fought each for nutrients and produced too many toxins. I thought for ages it was the Velcro or the glue killing em’.
And not only did they not die….they twitched! The muscle fibres twitched!
So now as in all research it’s time to repeat the experiment. A lot. To see if I can replicate it and analyse what I have.
Then try and put it into our thicker 3D system, this is super exciting!
On the out of lab experiences I really do love chatting science, tech, nature and food too and showing how balanced ecosystems can incorporate tech too. And I was lucky enough to be invited to the Science Gallery Dublin teamed with Kat and Zack from The Centre of Genomic Gastronomy and Oron Catts of Symbiotica for ArtMeatFlesh. A cookery show whereby artists, scientists and philopsophers discuss cellular agriculture.
I saw this as a prime was to open discussion about current farming methods how cultured meat can be part of a balanced ecosystem.
Our menu? We developed with real meaning behind it to highlight cellular agriculture prospects and also our current food system.
Team: Molly Garvey (Chef), Zack Denfeld (Artist/Designer), Abi Glencross (scientist)

Figure 15: Zack and I (apparently I pull a bizarre range of facial expressions…)
Amuse Bouche: Former food fantasies including insects, urban mushrooms and spirulina (algae).
Looking into fads that have again been popularised in order to address the ‘need’ for protein.
Starter: Feeding our food – Corn polenta, soy beans, lambs heart
The conversation centred on grain subsidies and the quantity and quality of grain going into animal feed, what would be needed for cultured meat and how these raw materials are being produced.

Figure 16: some of my arty side coming out in the decor
Main: The Small and Many – A variety of diverse charcuterie all of which can be traced back to the farm.
We are often quick to condemn animal products, however are we aware of the how diversity influences our countryside, cuisine and culture? What would happen if we asked the hard origin questions when we ate meat, if animals were removed from our ecosystem, or if saw cuts such as heart and bone as edible again? How could cellular agriculture compliment ecoagricultural farming and meat processing?
Dessert: Meat Layer Cake
Did you know that animal’s internal composition changes with the seasons? In winter they naturally have more fat to keep warm, and less fur in the summer. However meat legislation does not change, meaning farmers are restricted in what their product can contain meaning more waste and more raw materials for less output.
Also what if cellular agriculture meant we could change these compositions? Salami is made from taking whole cuts of meat deconstructing and reconstructing them. What if Cell Ag could make these selected cuts with less waste?
This kind of platform is amazing for opening the public’s mind to cultured meat and also looking back on how we grow, process and consume food now.
It was an amazingly exhilarating experience! I learnt so much, and I hope I passed on some knowledge to others too. It also highlighted how quickly people are to condemn the transparency in science, which I believe is changing with the new age of scientist. That why I do so much of this, to start the conversations before cultured meat hits the shelves as at the end of the day we all have to eat and are in this together.
Stay tuned for the video and ‘Diary of a Cell Famer – Part II’ soon!
Written by Abi Glencross, June 24, 2016
The Process
These are excerpts from Abi’s final project design proposal.
Official Project Title
Creation of In Vitro Thick 3D Vascularized Skeletal Muscle Tissue for the Production of Cultured Meat
Student
Abigail Glencross (MEng Chemical Engineering; University of Bath)
A recent graduate; Abi has worked on two hollow tube perfusion bioreactor projects and is accustomed to modelling mass, energy and flow configuration. After recently completing a six month cultured meat final year project which covered social, scientific and engineering aspects, Abi was employed by Bath University over the summer of 2014 to patent a cell scaffold for novel drug delivery. It was here she began building the necessary skills in scaffold characterization, cell culture and lab etiquette. She was also a member of the Royal Academy’s Ingenious team, which showcased the social and scientific impact of cultured meat on future society.
Supervisors
Prof. Mark Post (Professor of Vascular Physiology; Maastricht University)
Professor of vascular physiology, angiogenesis and head of the cultured meat project at Maastricht University; Mark has taken the first step in creating a viable meat source that will decrease worldwide dependency on livestock farming. Mark has extensive vascularisation and meat biology knowledge. Additionally he has been involved in numerous social engagement activities including TED talks and university seminars.
Prof. Lucy Di-Silvio (Cell Biologist; Kings College London)
Lucy’s expertise lies in tissue engineering, stem cell approaches for tissue regeneration and biocompatibility of materials. Lucy has in depth knowledge of cell-material interactions, environmental niches for cell differentiation and tissue regeneration which are paramount in replicating the cell biology of conventional meat.
Colleges
Kings College, London; Maastricht, NL
Duration
Full time; 3 years funded; 1 year optional extra for completion.
Rationale
The University of Maastricht has proved from adult bovine skeletal muscle stem cells it is possible to create edible cultured meat in a self-organised cell culture. This thin tissue (<0.5mm) can then be utilised to form ‘processed’ meats. These meats are fashioned from offcuts of the farming industry, however the vast environmental and ethical impacts of conventional meat are derived from whole primary animal products e.g. steak. In order to mitigate these negative effects thick 3D tissue must be produced. This requires synthetic vascularization to successfully facilitate oxygen and nutrient flow to, and metabolite removal from, the innermost cells to prevent necrosis. Currently self-organised tissues are limited by diffusional mass transport of these elements, and novel concepts of sustaining the innermost cells of >0.5mm tissues must be addressed.
Literature Review
Meat is comprised predominantly of water, muscle and fat. Therefore a coculture of myocytes (to form skeletal muscle fibres) and adipocytes (to form fat tissue) are required to replicate the taste, texture and aesthetic of conventional meat[i].
These cells require different optimal culture conditions, and coculture of multiple cell types have been successful in maintaining cell viability both in vitro and in vivo[ii]. There is often no outright consensus for optimal coculture conditions[iii] therefore it is the aim to establish this for cells of bovine origin.
In vitro vascularization is a major hurdle in thick 3D tissue engineering[iv] with several factors to consider. Aligned skeletal muscle engineering has been previously explored with success in thin 3D culture when cellular demands are not limited by nutrient, oxygen and metabolite diffusion[v]. When scaling up to thick 3D culture an artificial capillary system must be created and sustained to maintain the innermost cells and prevent tissue necrosis.
One method of creating a suitable perfusion system is to seed cells onto a permeable, degradable and channelled biomatrixxii. This scaffold can be in the form of hollow tubes which imitate a vascular network[vi] and degrade over time to form an edible product. Alternatively endothelial cells (EC) can be cultivated in the bioscaffold tube lumen to produce a vascular network mimicking in vivo. The paracrine effect of ECs on myocyte and adipocyte growth, differentiation and maturation remains unknown and will be studied.
The taste, texture and aesthetic of meat are highly affected by exercise induced hypertrophy. The differences between native and tissue engineered skeletal muscle metabolism have been studied[vii], however there is yet to be any empirical data for oxygen and nutrient uptake in thick 3D skeletal muscle under varying degrees of stimulation and metabolic demand. This study will assist in understanding the changing demands of skeletal muscle tissue when exercised.
Objective and Work Plan
Objective 1: To determine the optimum conditions for coculture of bovine endothelium, myocytes and adipocytes.
Culturing multiple cell types is challenging and requires a complex approach. Certain parameters can be set from knowledge attained from previous research. For example studies have shown that DMEM is a successful culturing medium for myocytes[viii], adipocytes[ix] and endothelial[x] cells. However, differentiation of these cells requires specific culture attributes such as media concentration, flow conditions, nutrients and define growth factors. Differentiation of endothelial cells, myocyte and adipocyte progenitors require specific phenotype stimuli that need to be carefully dose timed, singly or in combination. The variables to be optimized include: time between seedings, differentiation conditions, myocyte/adipocyte density/maturity upon seeding, overall culture time and bioreactor flow configuration. The paracrine effects of ECs will be studied by introducing controls with channels without ECs present.
Cell growth and differentiation will be verified for: myoctyes using histology or PCR for various markers of differentiation; adipocytes where fat content (oil-red-o, after elution of fat) will be monitored; and for endothelial cells imunohistochemistry (CD31 and VE-cadherin) characterized. 2 photon confocal imaging will allow cytoskeletal structures, reorganization and cell types to be tracked.
Objective 2: To use a manufactured 3D scaffold of hollow tube configuration for coculture and study its long-term integrity and stability.
A hollow tube will be constructed (3D printing/extrusion/etching) using collagen as a bioscaffold. Flow and pressure of medium through the lumen and extra cellular space (ECS) will be introduced (Fig 1) in an open shell forced-circulation loop flow configuration.
Myocytes will be seeded in a hydrogel surrounding the hollow tube and ECs through the lumen (Fig 1: ECS). Myocyte density and timing of differentiation will be optimized by seeding myocytes at different stages of differentiation. Spacing of anchors for myocytes will be defined and optimized. After myotube formation is successful this process will be repeated for adipocytes with the addition of long chain fatty acids to the media to induce differentiation. Adipocyte density, differentiation seeding stage and overall culture time will also be optimized.
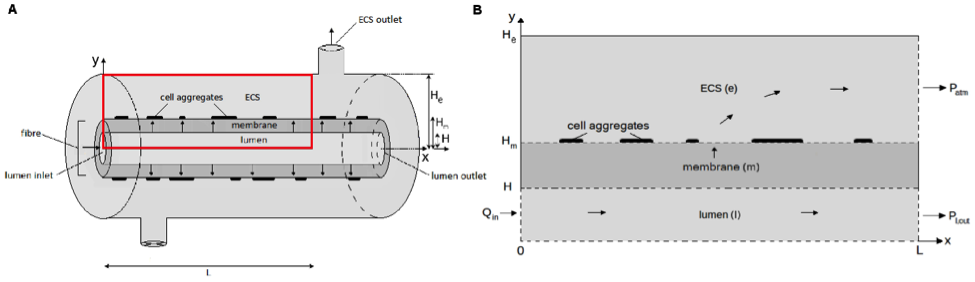
Figure 1: A Single Hollow Tube Schematic (Chapman et al., 2014)
Spatial oxygen and nutrient concentration profiles within the construct will be computed through modelling to determine optimum culture conditions and tube dimensions. Long-term integrity and stability of the tube vis-à-vis tissue formation will be monitored.
Objective 3: To develop a functional, reproducible and scalable organized vascular network.
There are two possible configurations to be explored for organized systems using the hollow tubes indicated in ‘Objective 2’: 1) Multiple Parallel, where tubes are not necessarily interconnected; and 2) Hierarchical Branching System, a system of interlinked tubes connected to a larger inlet and outlet tube attached to a perfusion system.
In both approaches the skeletal muscle cells are thought to self-organise within the manufactured construct. The first configuration would be initially implemented with tube diameter to length ratio optimized (‘Objective 2’) in terms of successful component (oxygen, nutrient and metabolite) mass transfer. In addition, an inflow and outflow hook-up will be designed and constructed to allow perfusion.
The configuration must also give rise to the second functionality of the tissue; the ability to mimic meat. Therefore appropriate texture and aesthetic are paramount.
Objective 4: To study the effects of stimulation on cell and tissue metabolism, oxygen consumption and splicing factors.
The culture will be exercised using electrical stimulation using parameters that were tested earlier in the work of Langelaan and of Boonen[xi],[xii],[xiii]. After this configuration has been established more energy efficient stimulations will be explored to induce contraction of the skeletal muscle tissue. The study will include the measure of nutrient and oxygen uptake; evolution of physical appearance; actin and myosin protein expression; and splicing factor expression in the engineered tissue.
Expected Output
Overall Output: A replicable, scalable and sustainable beef tissue with organized vascularization which successfully facilitates oxygen and nutrient transfer to, and metabolite removal from, the innermost cells. The product will be suitable for consumption and resemble beef in taste, texture and composition.
Milestones
1) A hollow tube with: endothelium cultured on the inner lumen; and with myocytes and adipocytes cultured in hydrogel in the extra capillary space.
2) A network of these tubes either interconnected or running in parallel. These tubes will successfully facilitate nutrient and oxygen transfer to, and metabolite removal from, cells within the tissue to prevent tissue necrosis.
3) A mathematical model for nutrient and oxygen transfer in single and multiple tubes with results on how engineered tissue metabolism varies with stimulation.
4) Public engagement which will include presentations at appropriate conferences and meetings.
5) A thesis detailing: optimum bovine endothelium, myocyte and adipocyte coculture conditions; a configuration for producing a successful organized vascular network in a 3D tissue; and how this engineered tissue changes with stimulation. Parts of this thesis will become scientific publications in international peer reviewed journals.
[i] Langelaan, M.L.P., Boonen, K.J.M, Polak, R.B., Baaijens, F.P.T., Post, M.J., & van der Schaft, D.W.J. (2010). Meet the new meat: tissue engineered skeletal muscle. 21(2), 59-66.
[ii] Xu, T., Zhao, W., Zhu, J. M., Albanna, M. Z., Yoo, J. J., Atala, A. (2013). Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials, 34(1), 130-139.
[iii] Baldwin, J., Antille, M., Bonda, U., De-Juan-Pardo, E. M., Khosrotehrani, K., Ivanovski, S., Petcu, E. B., Hutmacher, D. W. (2014). In vitro pre-vascularisation of tissue-engineered constructs a co-culture perspective. Vasc Cell, 6, 13.
[iv] Ko, H. C., Milthorpe, B. K., McFarland, C. D. (2007). Engineering thick tissues–the vascularisation problem. Eur Cell Mater, 14, 1-18.
[v] Bian, W., Bursac, N. (2009). Engineered skeletal muscle tissue networks with controllable architecture. Biomaterials, 30(7), 1401-1412.
[vi] Meneghello, G (2010). Development of a Novel PVA-PLGA Hollow Fibre Bioreactor for Tissue Engineering. Thesis (Doctor of Philosophy (PhD)). University of Bath.
[vii] Cheng, C. S., Davis, B. N., Madden, L., Bursac, N., Truskey, G. A. (2014). Physiology and metabolism of tissue-engineered skeletal muscle. Exp Biol Med, 239(9), 1203-1214.
[viii] Dodson M.V., Martin E.L., Brannon M.A., Mathison B.A, McFarland D.C. (1987). Optimization of bovine satellite cell-derived myotube formation in vitro. Tissue and Cell, 19(2), 159-166
[ix]Wei, S., Du, M., Jiang, Z., Duarte, M., Fernyhough-Culver, M., Albrecht, E., Will, K., Zan, L., Hausman, GJ., Elham M,. Elabd, Y., Bergen, WG., Basu, U., Dodson, MV. (2013). Bovine dedifferentiated adipose tissue (DFAT) cells. Adipocyte, 2(3), 148-159.
[x] Folkman, J., Haudenschildf, CC,. Zetter, BR,. (1979). Long-term culture of capillary endothelial cells. Proc Natl Acad Sci, 76(10), 5217-5221.
[xi] Langelaan ML, Boonen KJ, Rosaria-Chak KY, van der Schaft DW, Post MJ, Baaijens FP (2012). Advanced maturation by electrical stimulation: Differences in response between c2c12 and primary muscle progenitor cells. Journal of tissue engineering and regenerative medicine. 5:529-539
[xii] Boonen KJ, van der Schaft DW, Baaijens FP, Post MJ (2011). Interaction between electrical stimulation, protein coating and matrix elasticity: A complex effect on muscle fibre maturation. Journal of tissue engineering and regenerative medicine. 5:60-68
[xiii] Boonen KJ, Langelaan ML, Polak RB, van der Schaft DW, Baaijens FP, Post MJ (2010). Effects of a combined mechanical stimulation protocol: Value for skeletal muscle tissue engineering. Journal of biomechanics. 43:1514-1521